
In vitro, primary astrocyte cultures express iNOS in response to cytokines such as IL-1β, interferon γ (IFNγ), TNFα and/or the bacterial endotoxin, lipopolysaccharide (LPS). The expression of iNOS can be induced in different cell types and tissues by exposure to immunological and inflammatory stimuli. NO, one of the smallest known bioactive products of mammalian cells, is biosynthesized by three distinct isoforms of NO synthase (NOS): the constitutively expressed neuronal (n)NOS and endothelial (e)NOS, and the inducible (i)NOS. Reactive astrocytes are capable of producing a variety of pro-inflammatory mediators, including interleukin-6 (IL-6), IL-1β, tumor necrosis factor-α (TNF-α), neurotrophic factors, as well as potentially neurotoxic compounds, like nitric oxide (NO). Together with the marked anti-inflammatory effects that we previously observed in microglial cells, these data suggest possible beneficial effects of mTOR inhibitors in the treatment of inflammatory-based CNS pathologies.Īstrocyte activation has been implicated in the pathogenesis of several neurological conditions, such as neurodegenerative diseases, infections, trauma, and ischemia. The present findings show that mTOR controls the rate of iNOS mRNA degradation in astrocytes. Similarly, rapamycin induced a significant up-regulation of tristetraprolin (TTP), a protein involved in the regulation of iNOS mRNA stability. In this regard, we found that rapamycin significantly reduced the half-life of iNOS mRNA, from 4 h to 50 min when cells were co-incubated with cytokine mixture and 10 nM rapamycin. Interestingly, reduced levels of iNOS mRNA were detected after 48 hours, suggesting that rapamycin can modify iNOS mRNA stability.
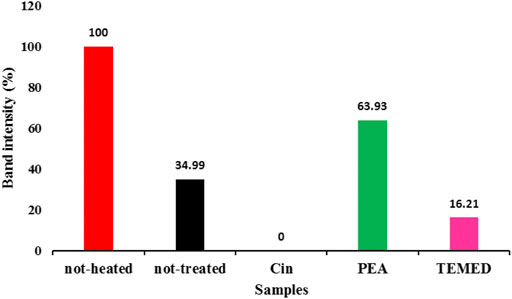
This stimulatory effect was transient, since no differences in either iNOS mRNA or protein levels were detected after 24 h. However, the drug significantly increased iNOS mRNA levels after 4 h incubation in presence of pro-inflammatory stimuli. Rapamycin did not display any significant effects under basal conditions, on either iNOS activity or its expression. In this experimental paradigm mTOR activation did not significantly affect astrocyte iNOS activity, but mTOR pathway was involved in the regulation of iNOS expression. Functional experiments to evaluate iNOS activity were also included.

Rapamycin was used at nM concentrations to block mTOR activity and under these conditions we measured its effects on the iNOS promoter, mRNA and protein levels.
The intensities of bands were determined by imagej software plus#
Primary cultures of rat cortical astrocytes were activated with different proinflammatory stimuli, namely a mixture of cytokines (TNFα, IFNγ, and IL-1β) or by LPS plus IFNγ. In this study the effects of mTOR inhibition on iNOS regulation were evaluated in astrocytes.

However, with respect to iNOS expression, both stimulatory and inhibitory actions involving the mTOR pathway have been described. It is also a key regulator of intracellular processes in glial cells. mTOR is a serine-threonine kinase that plays an evolutionarily conserved role in the regulation of cell growth, proliferation, survival, and metabolism. The mammalian target of rapamycin (mTOR) kinase modulates the activity of some proteins directly involved in post-transcriptional processes of mRNA degradation. The expression of iNOS is tightly regulated by complex molecular mechanisms, involving both transcriptional and post-transcriptional processes. High amounts of NO are synthesized following up-regulation of inducible NO synthase (iNOS). Reactive astrocytes are capable of producing a variety of pro-inflammatory mediators and potentially neurotoxic compounds, including nitric oxide (NO).


 0 kommentar(er)
0 kommentar(er)
